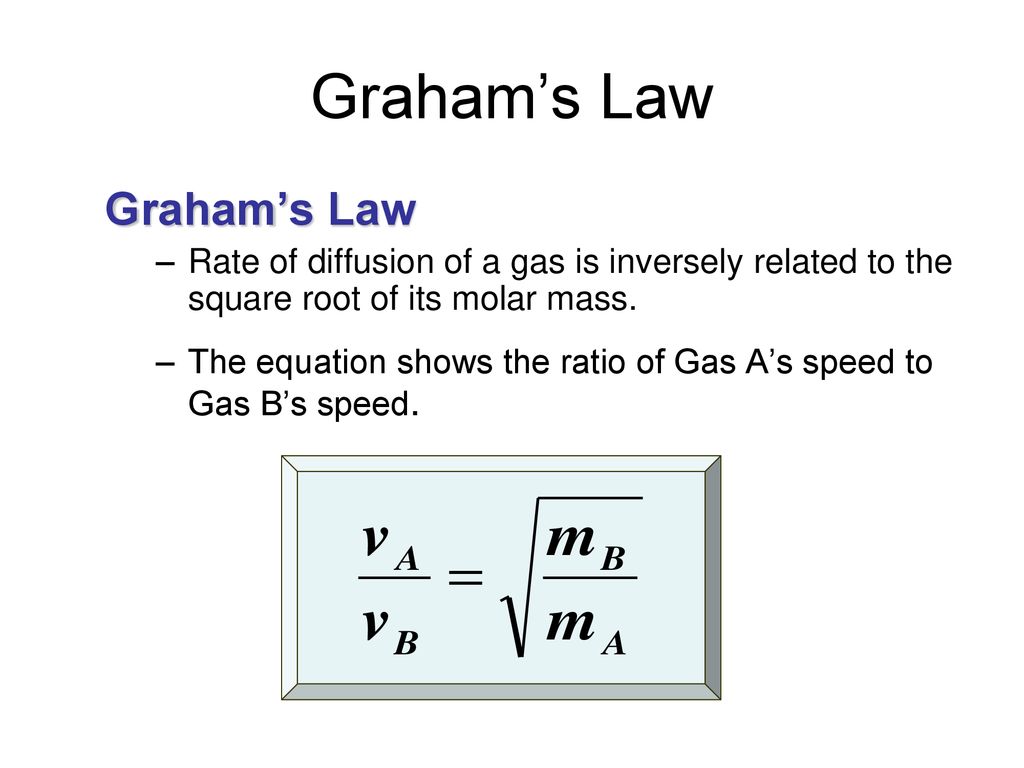
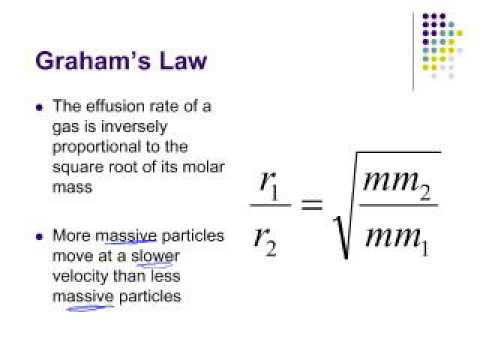
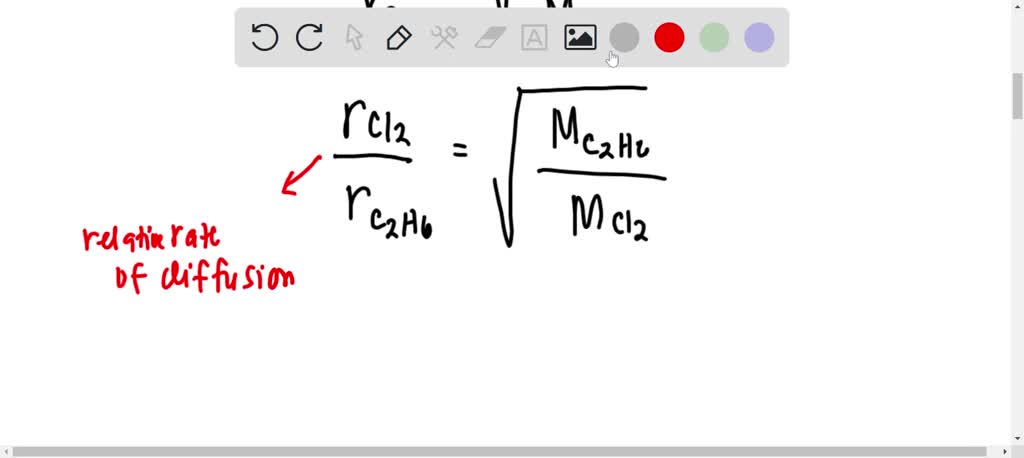
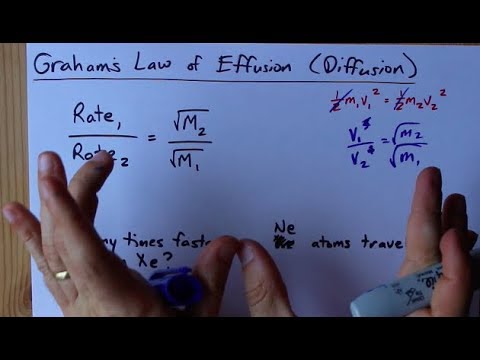
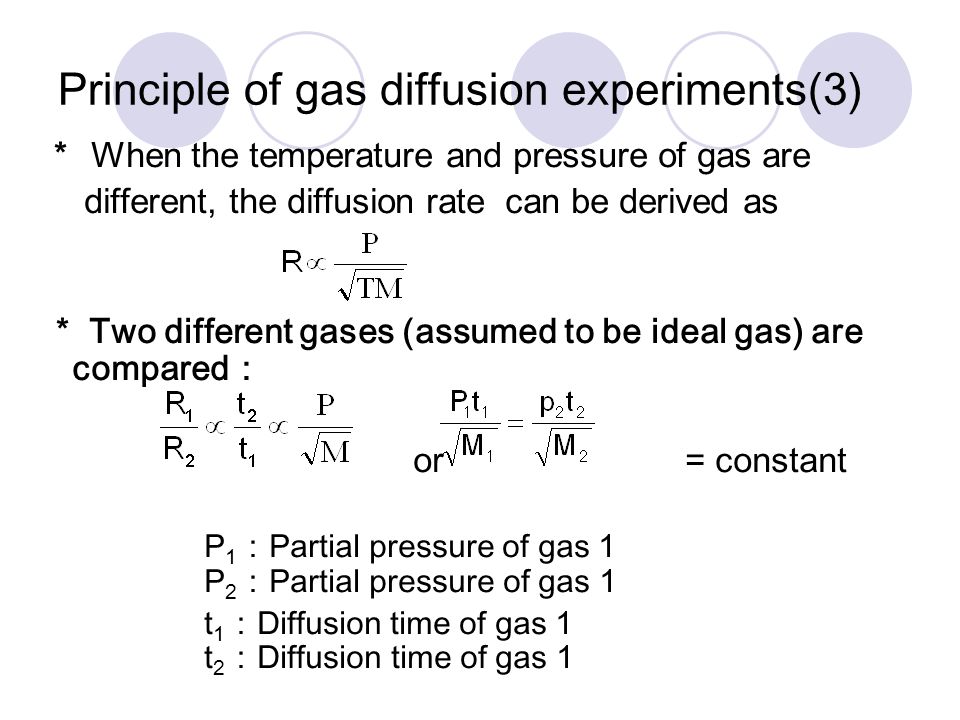
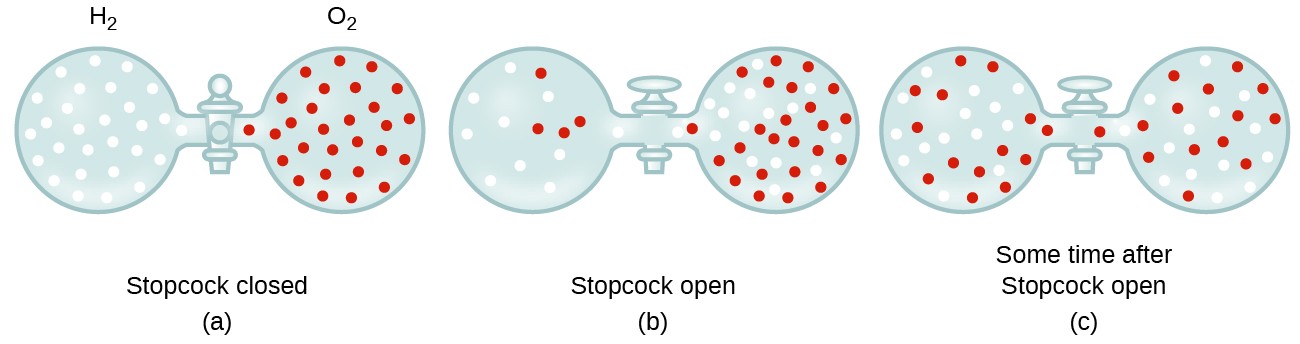
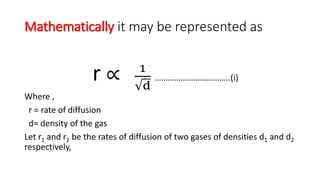
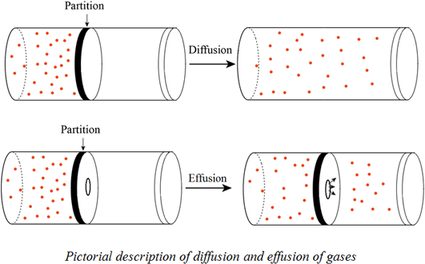
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora

The rate of diffusion of 2 gases 'A' and 'B' are in the ratio 16:3. If the ratio of their masses present in the mixture is 2.3. Then A) The ratio of
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be