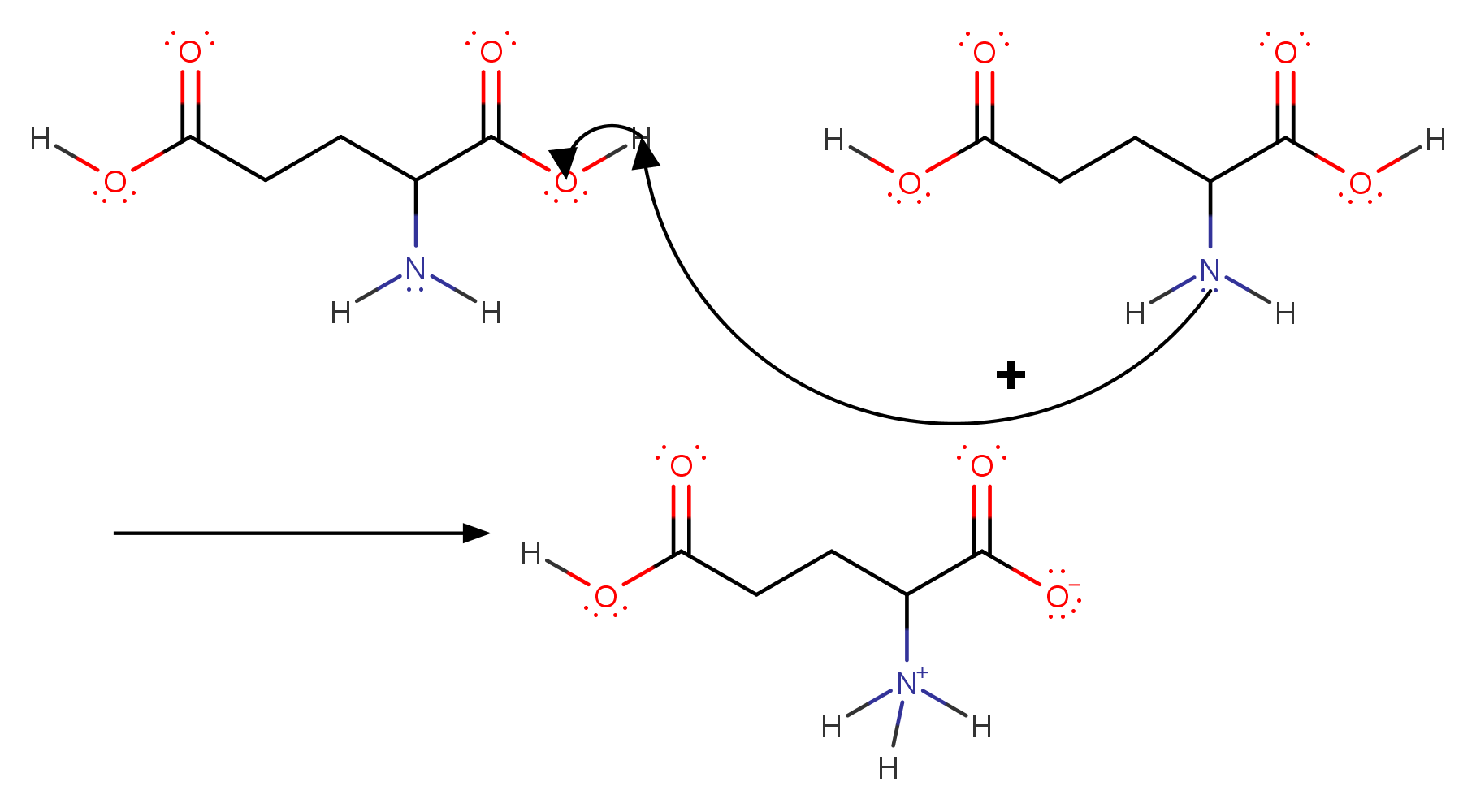
Scheme of synthesis for DEAE–CH derivatives; pK1, pK2 and pK3 indicate... | Download Scientific Diagram
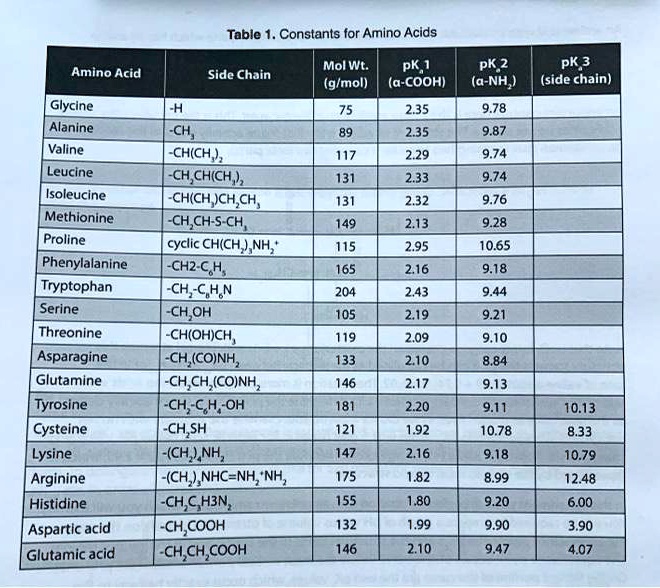
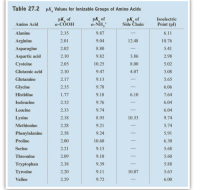
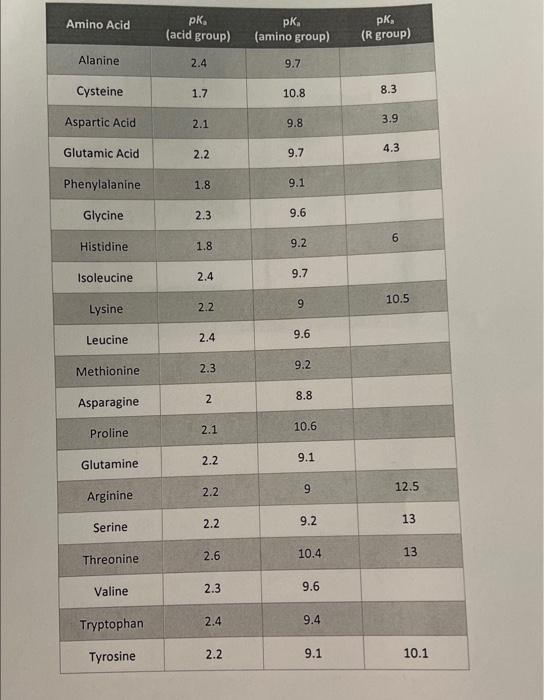
SOLVED: Table Constants for Amino Acids Mol WL: pK,1 (g/mol) (a-COOH) PK 2 (a-NH2) PK 3 Amino Acid Side Chain (side chain) Glycine Alanine 2.35 9.78 9.87 CH3 CH(CH3) CH(CH3)2 CH(CH3)2 CH2CH(CH3)2
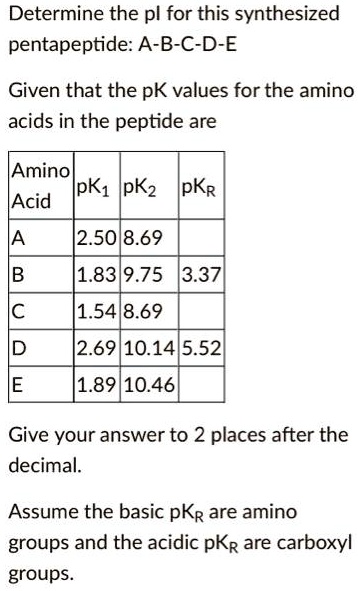
SOLVED: Determine the pI for this synthesized pentapeptide: A-B-C-D-E Given that the pK values for the amino acids in the peptide are: Amino acid pK1 pK2 pK3 Acidic pKR Basic pKR A

Safety Assessment of α-Amino Acids as Used in Cosmetics - Christina L. Burnett, Bart Heldreth, Wilma F. Bergfeld, Donald V. Belsito, Ronald A. Hill, Curtis D. Klaassen, Daniel C. Liebler, James G.

SOLVED: The pks for the ionizable groups in the amino acid lysine are pk1 = 2.18, pk2 = 8.95, and pk3 = 10.53. what would be the net charge on lysine at ph = 7.0
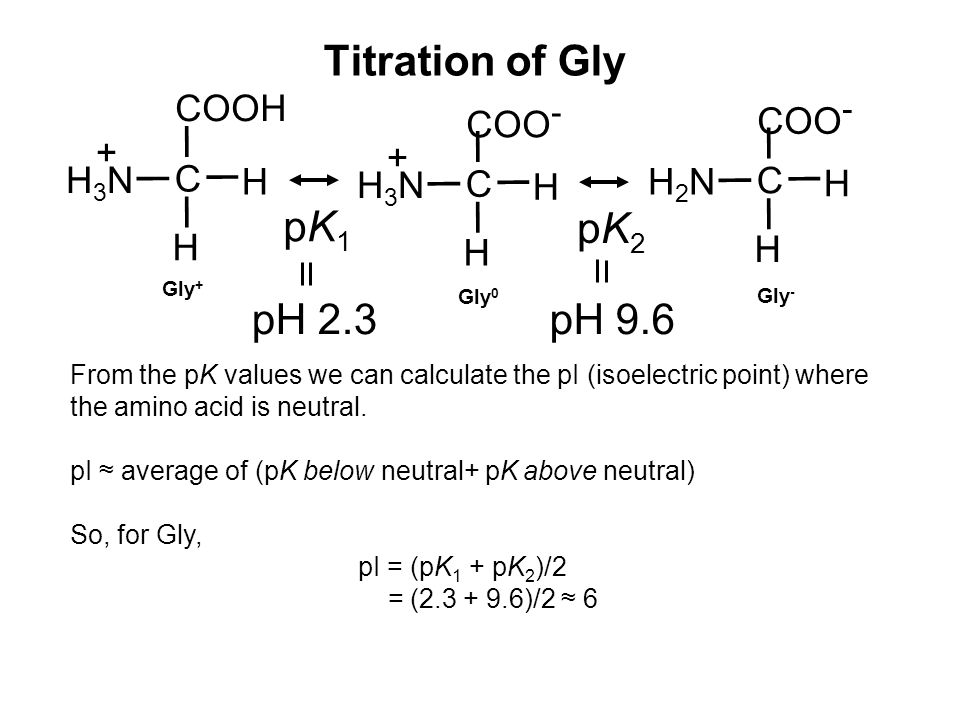
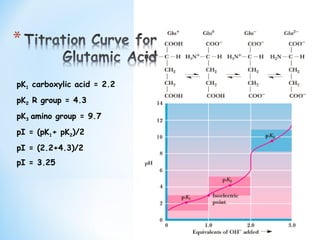
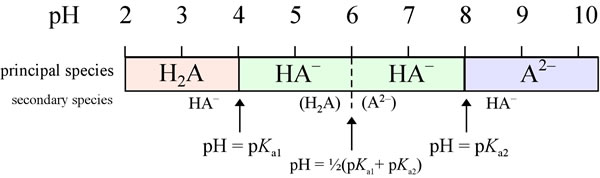
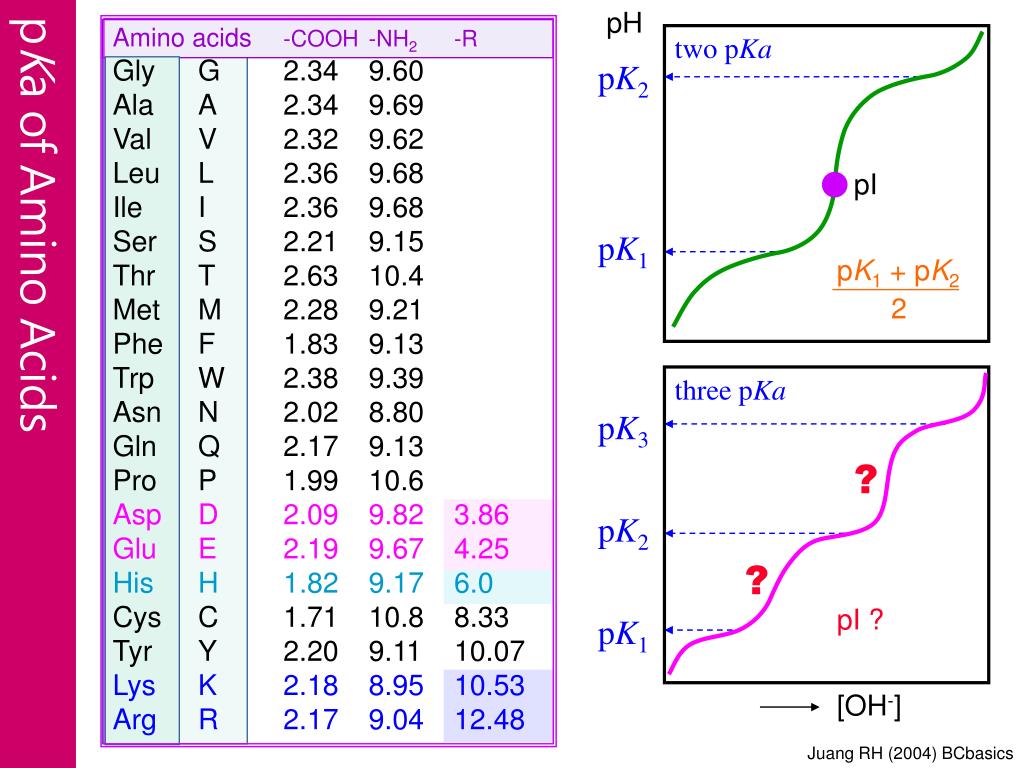
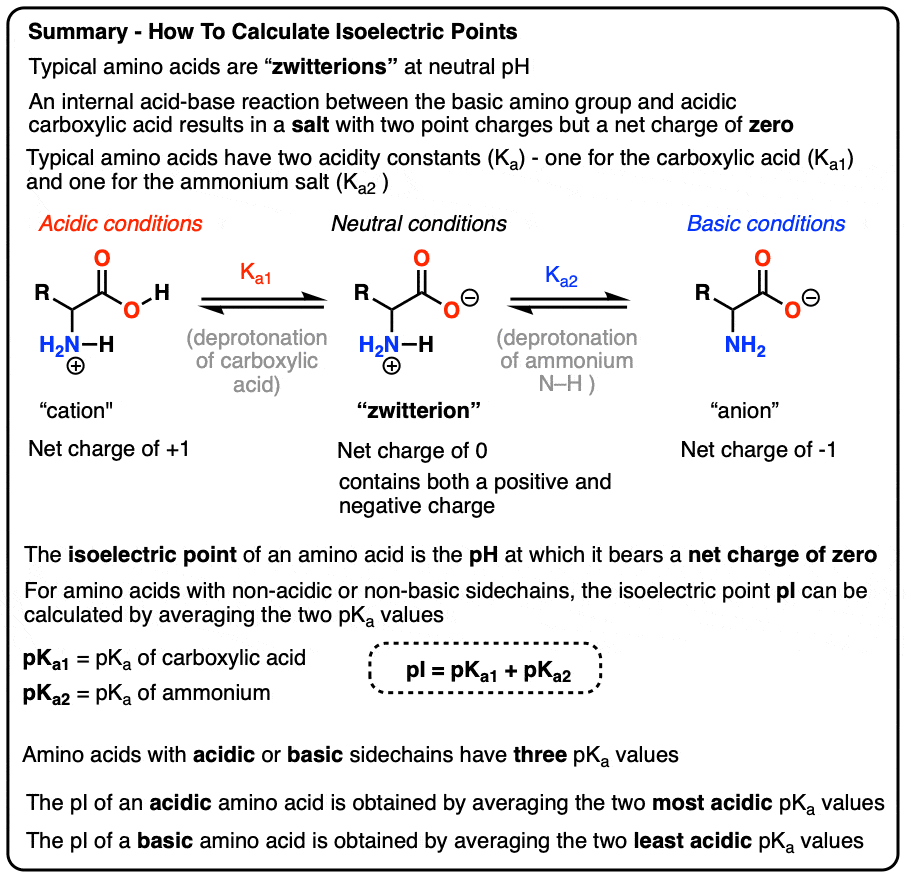
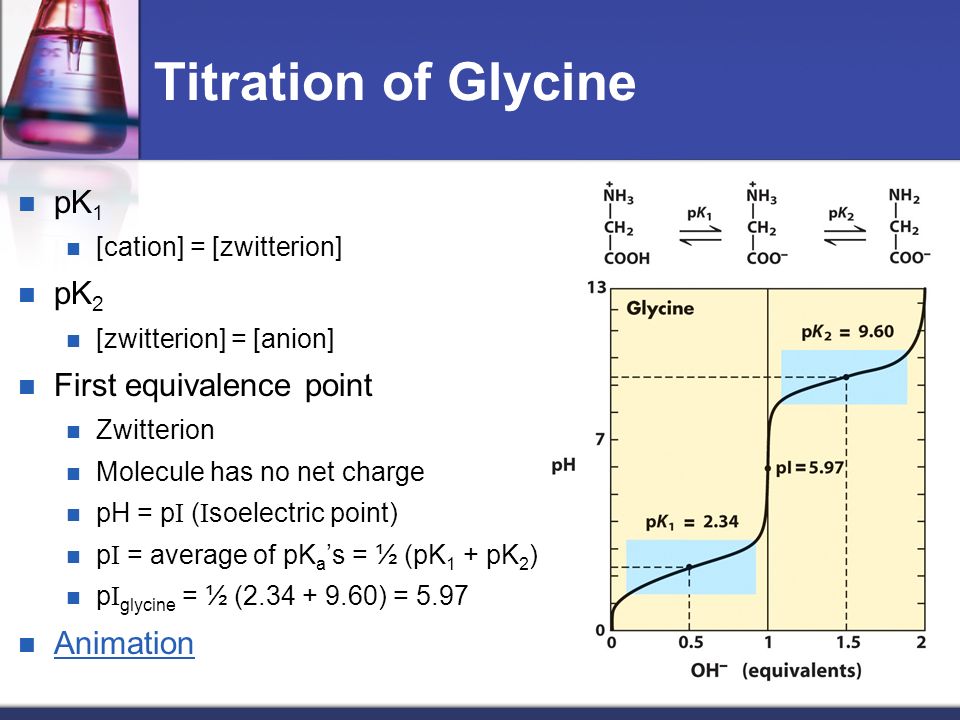
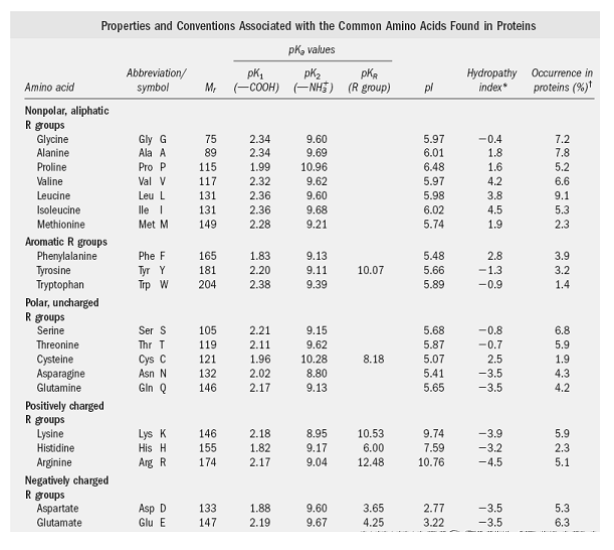
Lecture 3: Amino Acids Bonus seminar today at 3PM 148 Baker (bonus point assignment due on Wed. in class or electronically by ) Quiz next Wed. (9/7) - ppt video online download
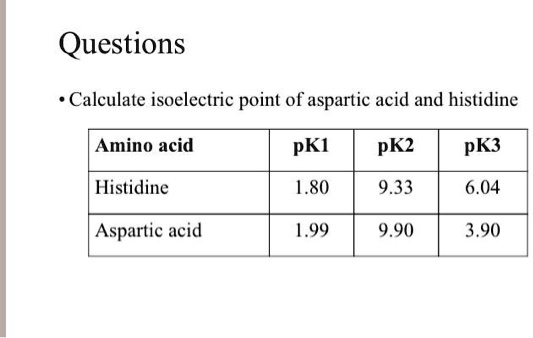
SOLVED: Calculate the isoelectric point of aspartic acid and histidine. Amino acid pKI pK2 pK3 Histidine 1.80 9.33 6.04 Aspartic acid 1.99 9.90 3.90

The amino acid methionine has pKa1 = 2.2 and pKa2 = 9.1. If this amino acid is represented by H2L+, what is the major species at pH 6? | Homework.Study.com
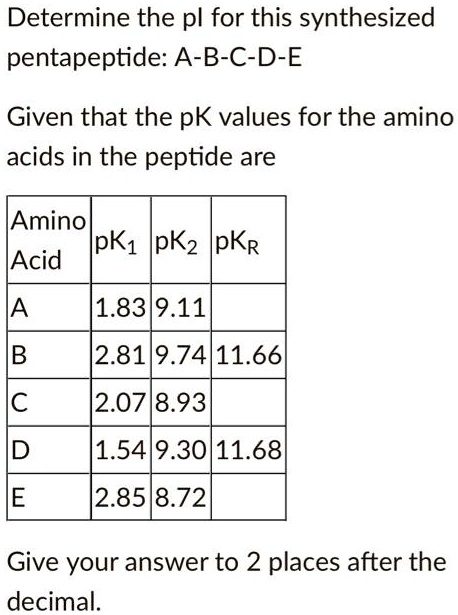
SOLVED: Determine the pI for this synthesized pentapeptide: A-B-C-D-E Given that the pK values for the amino acids in the peptide are: Amino Acid | pK1 | pK2 | pKR A

Scheme of synthesis for DEAE–CH derivatives; pK1, pK2 and pK3 indicate... | Download Scientific Diagram