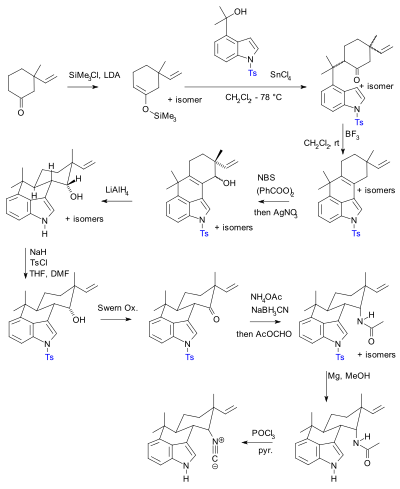
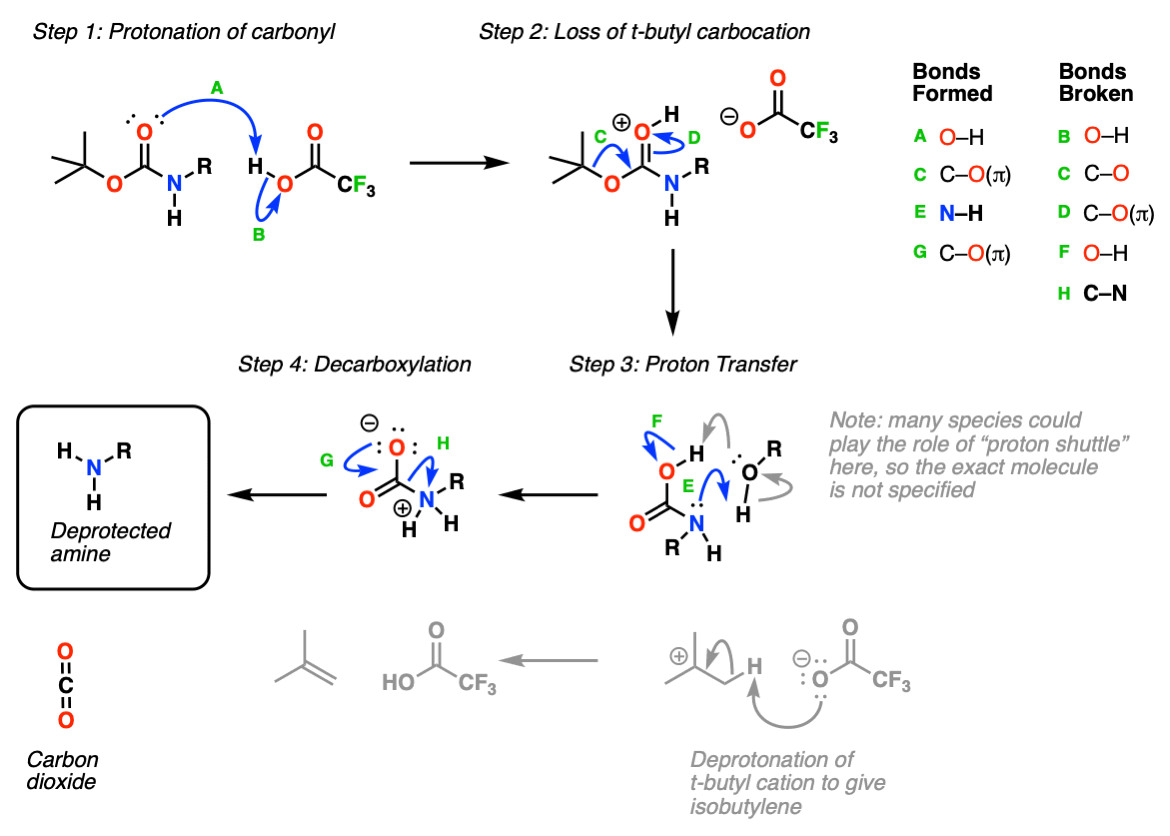
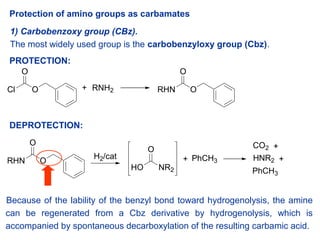
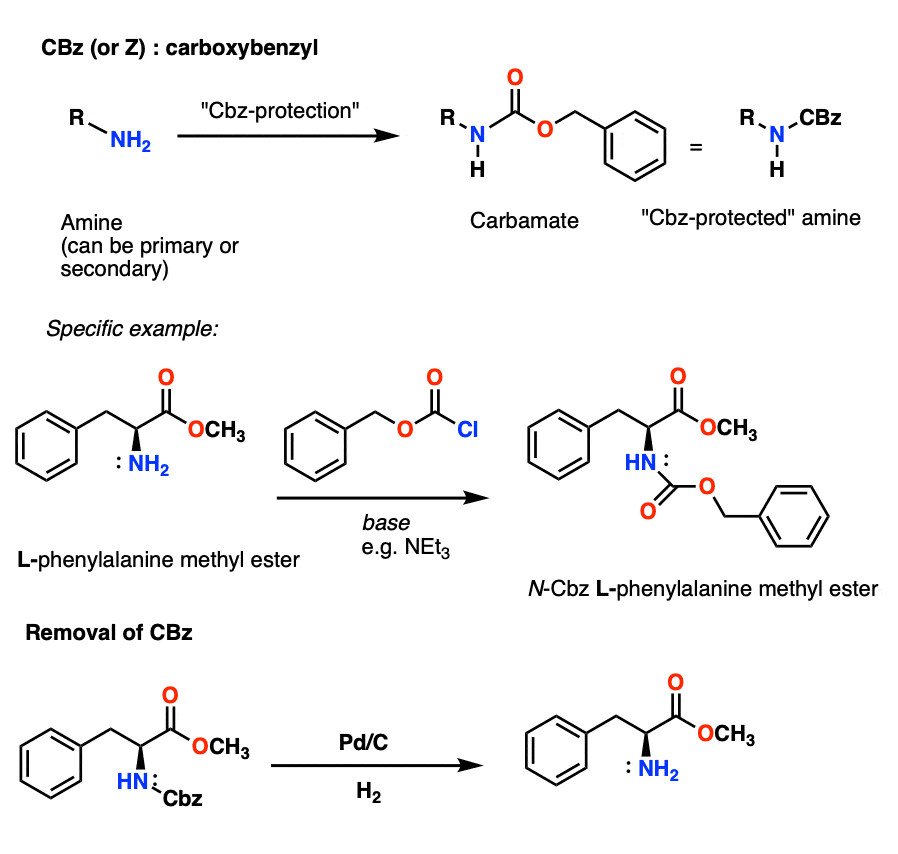
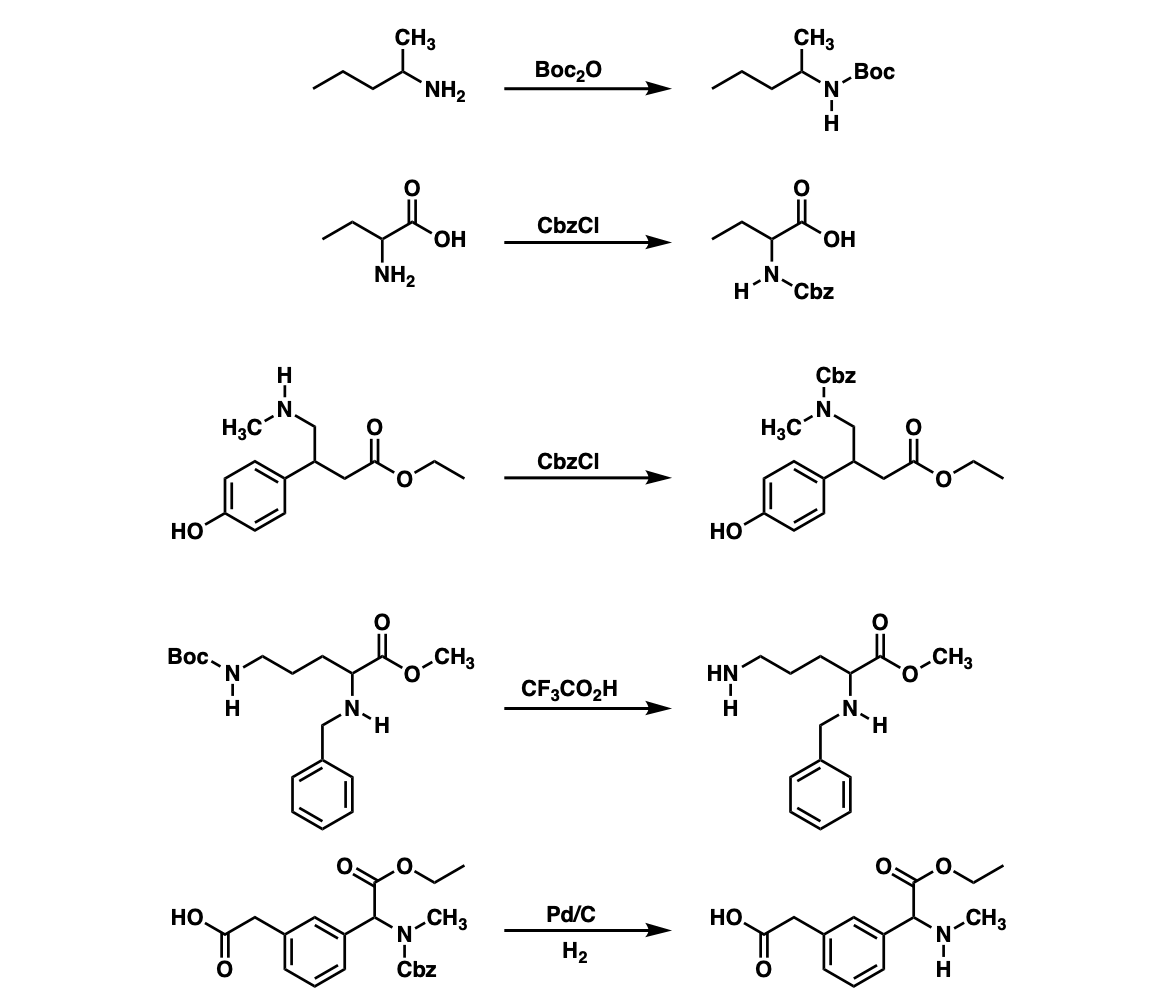
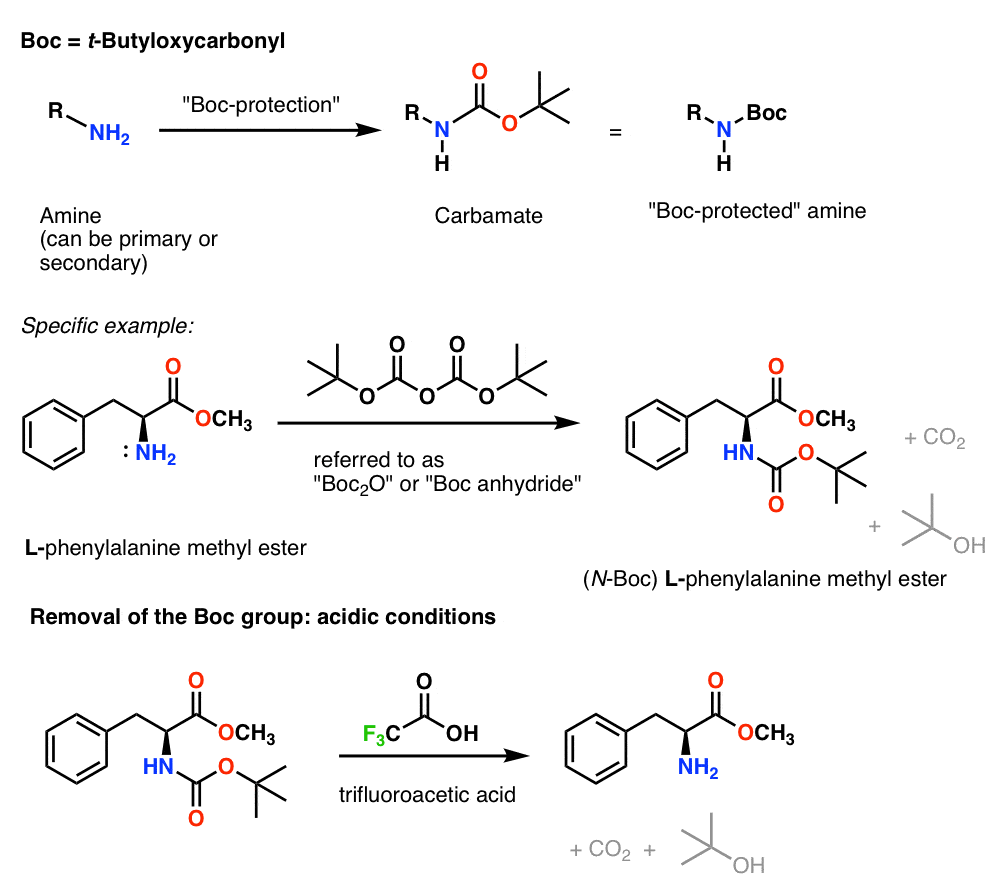
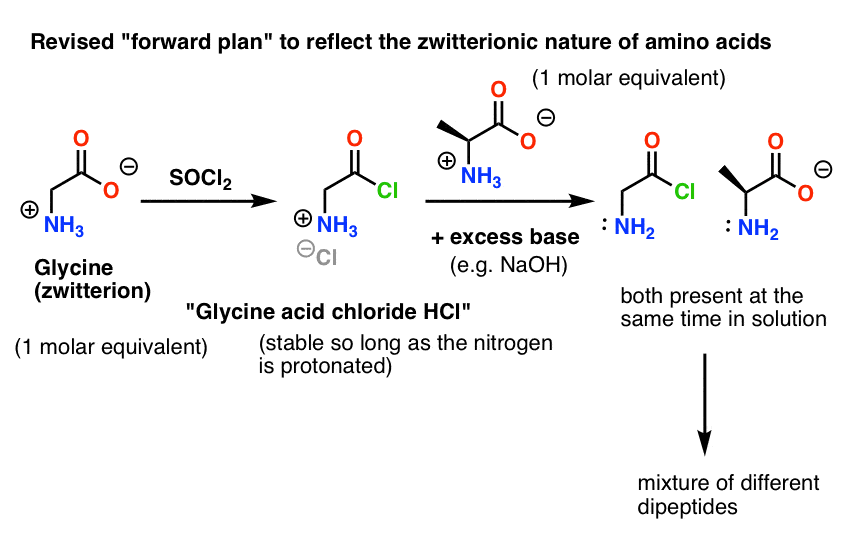
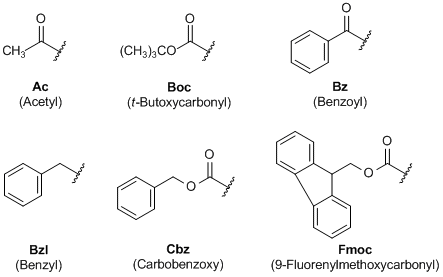
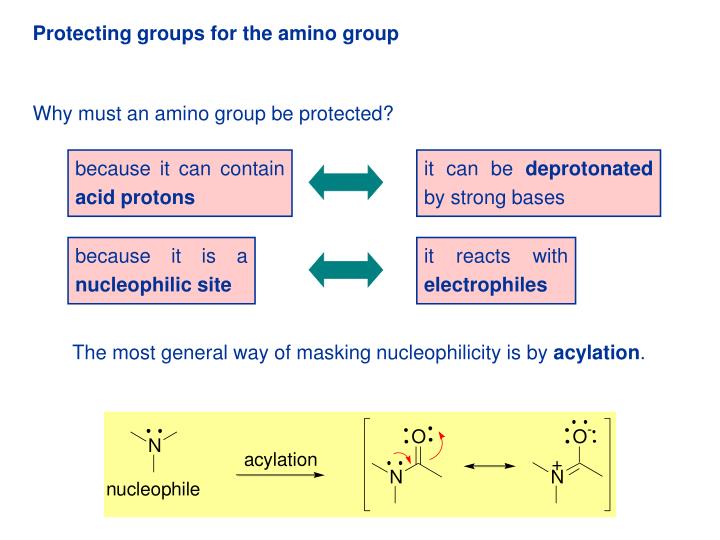
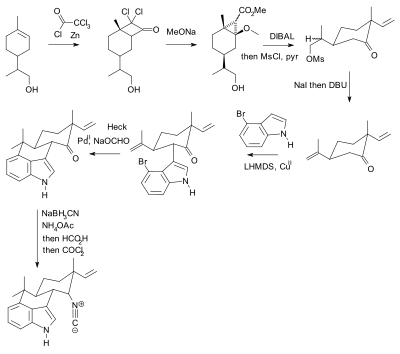
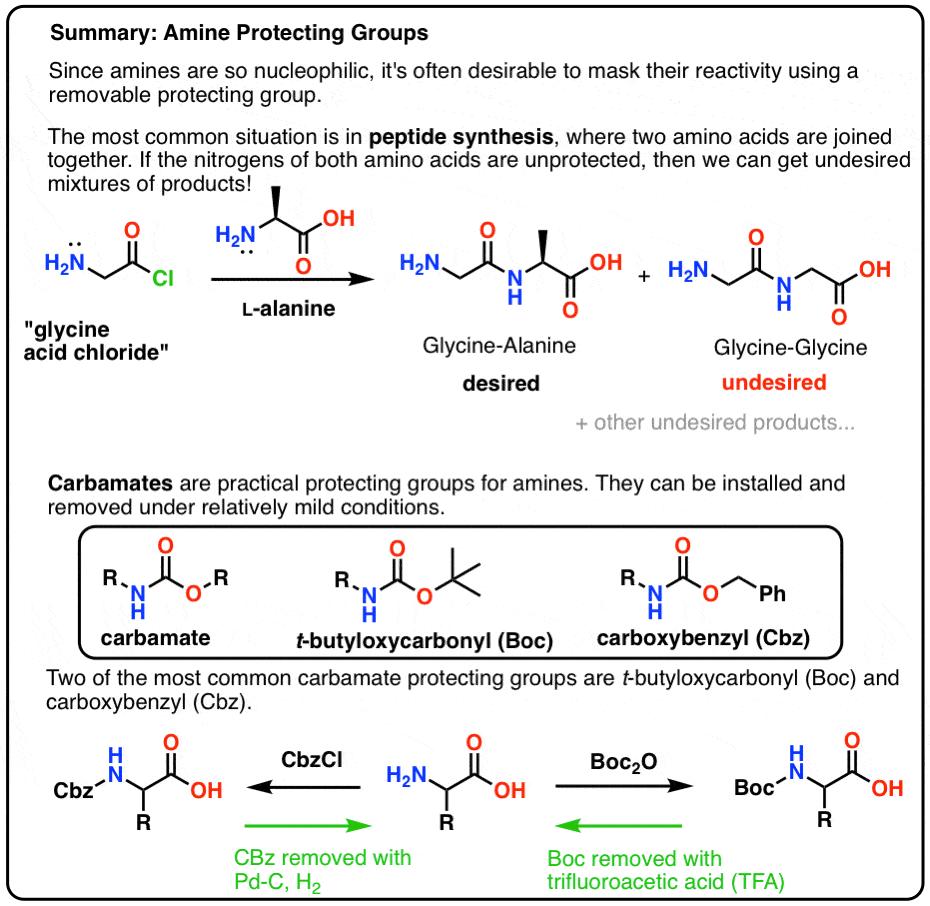
Selective Protection and Deprotection of Primary Amino Group Using 1,3-Dimethyl-5-acetylbarbituric Acid | Tokyo Chemical Industry (India) Pvt. Ltd.
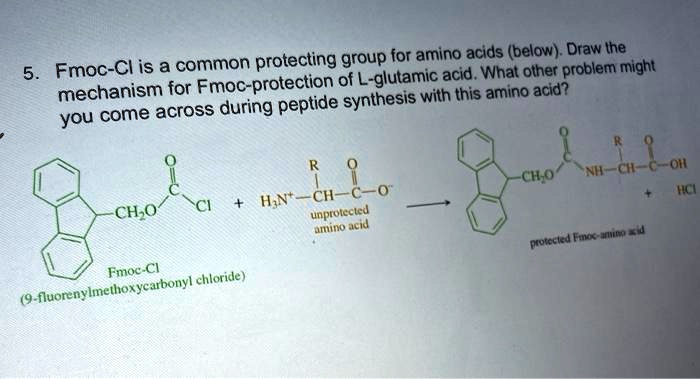
SOLVED: Fmoc-Cl is a common protecting group for amino acids. What other problem might arise with this amino acid during peptide synthesis?

Utilization of Fukuyama's sulfonamide protecting group for the synthesis of N-substituted α-amino acids and derivatives - ScienceDirect

Chemical structure and short names of TFA-labile protecting groups used... | Download Scientific Diagram
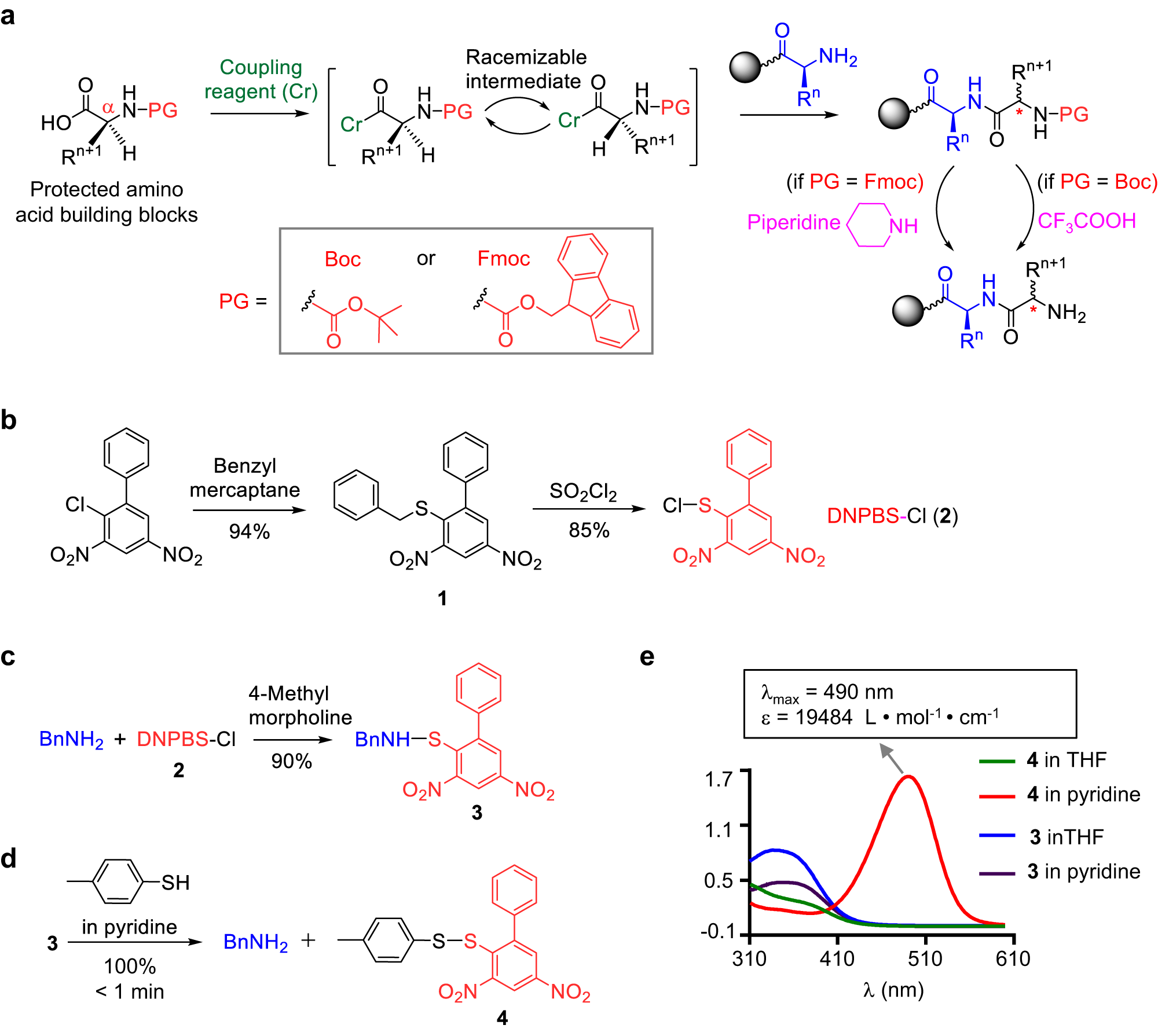
Suppression of alpha-carbon racemization in peptide synthesis based on a thiol-labile amino protecting group | Nature Communications

Unlocking the Potential of Phenacyl Protecting Groups: CO2-Based Formation and Photocatalytic Release of Caged Amines | The Journal of Organic Chemistry

Synthesis of Sulfonimidamide‐Based Amino Acid Building Blocks with Orthogonal Protecting Groups - Chinthakindi - 2019 - European Journal of Organic Chemistry - Wiley Online Library